Your privacy is very important to us. When you visit our website, please agree to the use of all cookies. For more information about personal data processing, please go to Privacy Policy.


What is Duchenne Muscular Dystrophy?
Duchenne muscular dystrophy (DMD) is a rare, fatal X-linked neuromuscular genetic disease caused by loss-of-function mutations in the DMD gene encoding the dystrophin protein. Mutations in the dystrophin gene can result in absence of insufficient functional dystrophin, a cytoskeletal protein that enables strength, stability, and functionality of myofibres1. Muscles without dystrophin are prone to damage, resulting in progressive loss of muscle mass accompanied by inflammatory muscular infiltration. This in together with cardiomyopathy2 reduces life expectancy.
DMD mainly affects boys, and it is the most common childhood muscular dystrophy with an incidence of ~1 in 3500-5000 live male births worldwide3.
Refs:
1.Birnkrant et al. Lancet Neurol. 2018. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management
2.Duan et al. Nature Reviews Disease Primers. 2021. Duchenne muscular dystrophy
3.Erkut et al. International Journal of Molecular Sciences. 2022. CRISPR Therapeutics for Duchenne Muscular Dystrophy
-
1 in 3500-5000live male births3

What Are the Symptoms Associated with DMD?
The earliest symptoms in DMD patients are difficulties with running, climbing stairs, jumping, getting up from the ground, falls frequently and develops a waddling gait at 3–5 years of age¹ due to the progressive muscle wasting and weakness. Most patients become wheelchair dependent around 10–12 years of age². They also subsequently develop respiratory insufficiency owing to the gradual loss of respiratory muscle strength and requiring ventilation assistance, as well as develop dilated cardiomyopathy in the late-teens or early 20s. Even with medical care, most people with DMD die from cardiac or respiratory failure before or during their 30s ³. In addition to striated muscle pathology, a proportion of DMD patients also develop a variable degree of intellectual disability or cognitive impairment⁴.
Crisafulli et al. Orphanet Journal of Rare Diseases. 2020. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis Duan et al. Nature Reviews Disease Primers. 2021. Duchenne muscular dystrophy Ryder et al. Orphanet Journal of Rare Diseases. 2017. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review Chey et al. WIREs Mechanisms of Disease. 2022. CRISPR applications for Duchenne muscular dystrophy: From animal models to potential therapies
How is DMD diagnosed?
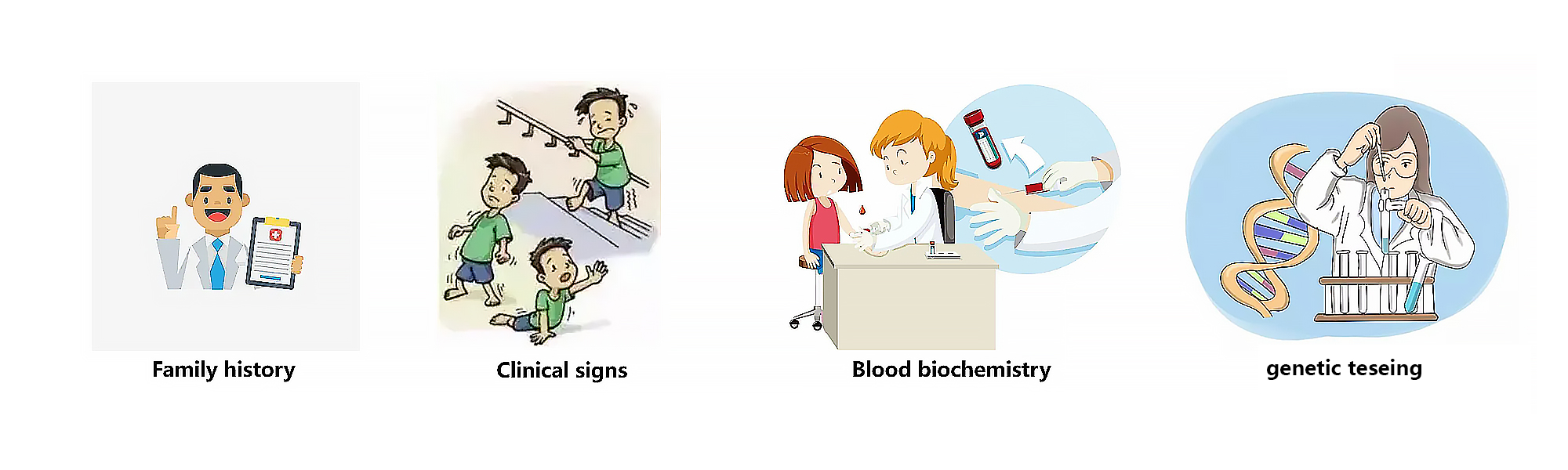
- If there is no prior family history of DMD, the diagnostic process typically begins in early childhood when suggestive signs and symptoms are noticed, such as weakness, clumsiness, a Gowers’sign, difficulty with stair climbing, or toe walking1. Besides, plasma CK should be measured in patients with symptoms of DMD as CK levels are generally substantially increased in patients with DMD from birth ² . In addition, it is crucial to identify the DMD mutation in those individuals to confirm diagnosis and a muscle biopsy sample should be tested for the presence of dystrophin protein if genetic testing does not confirm a clinical diagnosis of DMD¹ .
1.Birnkrant et al. Lancet Neurol. 2018. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management
2.Duan et al. Nature Reviews Disease Primers. 2021. Duchenne muscular dystrophy
What Are the Treatment Options for DMD?
-
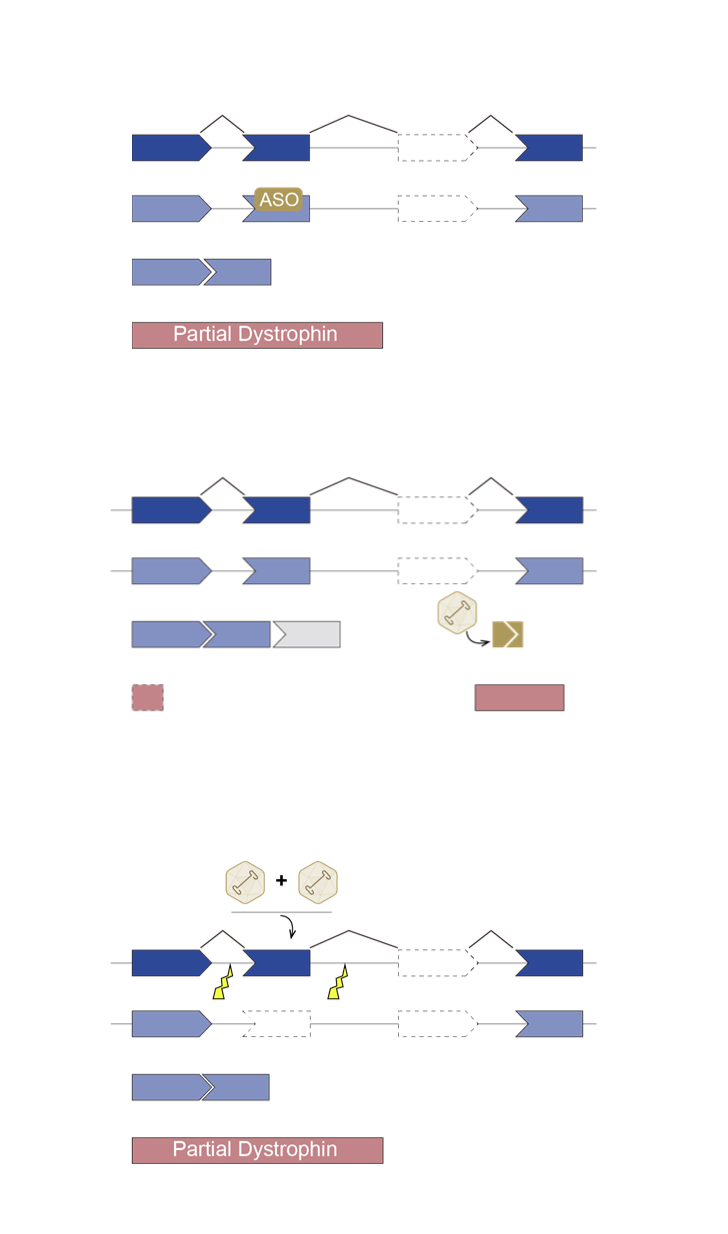
There is currently no curative treatment for DMD, although a multidisciplinary medical, surgical, and rehabilitative approach such as corticosteroids, physical therapy, and treatment of cardiomyopathy targeting the symptoms of DMD can delay disease progression and improve quality of life, thereby prolonging life expectancy for DMD patients ¹. In addition, many therapeutic strategies for DMD are in development that target dystrophin deficiency, such as gene replacement, exon skipping, stop codon readthrough and stem cell therapy, as well as strategies that target secondary pathology of DMD, such as anti-fibrotic, anti-inflammatory, antioxidant, or cardioprotective compounds². Furthermore, gene editing via clustered regularly interspaced short palindromic repeats (CRISPR) system is a promising therapeutic for DMD, as it can permanently correct DMD mutations that allowing for the production of functional dystrophin.
Previous : HG004(LCA)
Next : HG204(MDS)