Your privacy is very important to us. When you visit our website, please agree to the use of all cookies. For more information about personal data processing, please go to Privacy Policy.


HuidaGene Announces Completion of First Patient Enrollment in its Ophthalmic Gene Therapy Candidate HG004 IIT Clinical Trials
SHANGHAI and CLINTON, N.J., Apr. 10, 2023 - HuidaGene Therapeutics (HuidaGene), a global clinical-stage biotechnology company focused on developing genomic medicine, announced today that it has successfully completed the HG00401 clinical study "A study to evaluate gene therapy for RPE65 mutation in Leber's type 2 's congenital leukodystrophy (LCA2) with RPE65 mutation," the first patient was given a single ocular injection of the new gene therapy HG004.
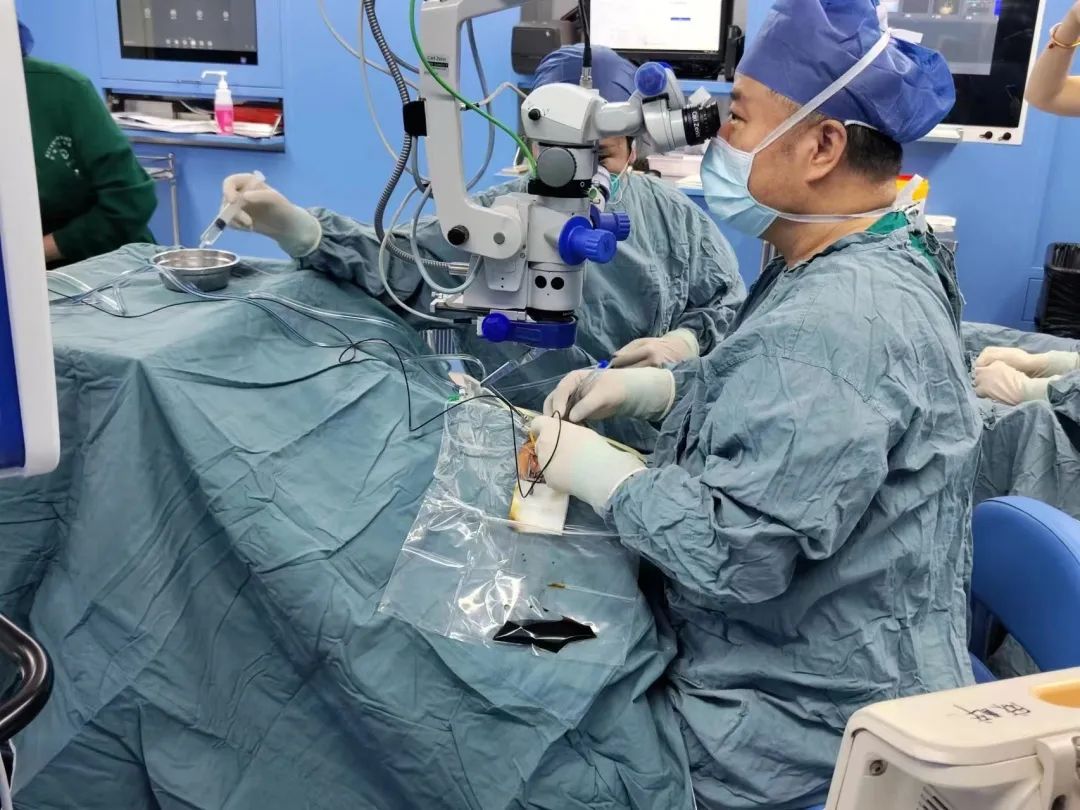
The patient was evaluated in detail before and after admission, and the preoperative preparation was improved, and the patient was administered by Director Peiquan Zhao. Within 2 weeks after low-dose HG004 administration, this subject had a good safety profile and no serious adverse events, ocular adverse reactions (no intraocular inflammation was observed), significant drug-related adverse events, or clinically significant immune reactions were observed. The patient's study eye showed rapid and sustained improvement in best-corrected visual acuity in addition to relevant visual function indicators (e.g., dynamic visual field, full visual field stimulation threshold). At the same time, the patient indicated that the study eye could see things that could not be seen before surgery even in cloudy or dim environment, which gave him a great confidence to look forward to a more convenient life in the future.
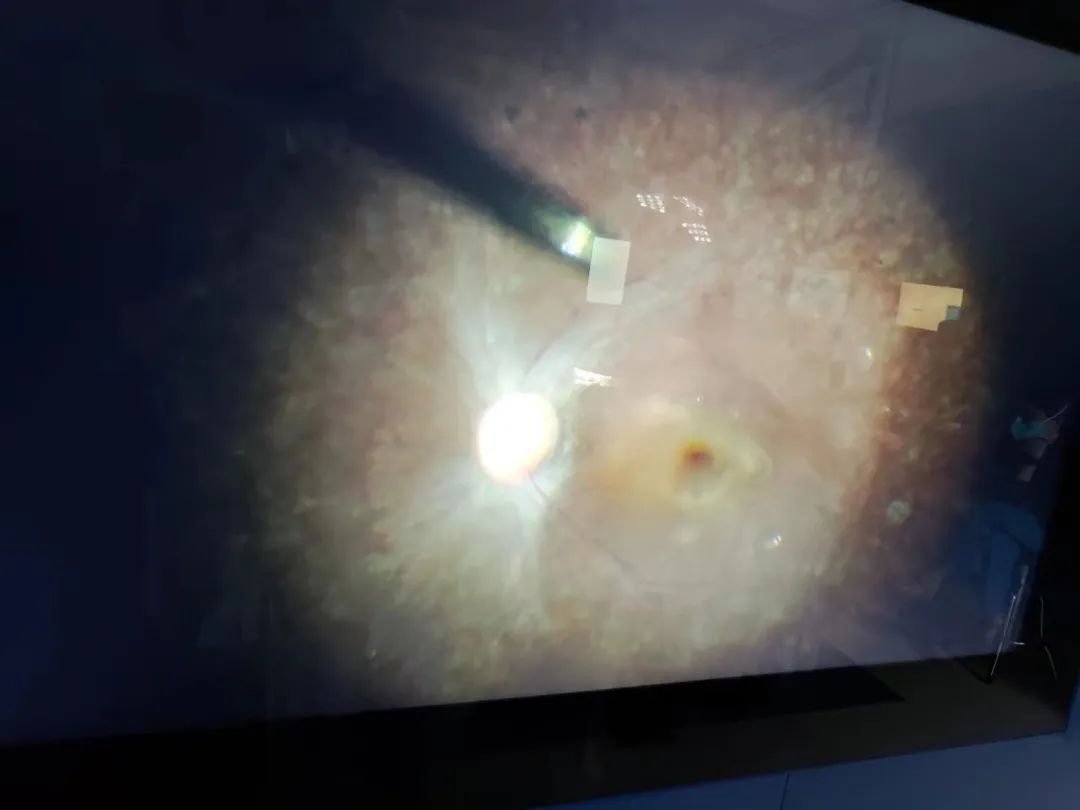
This HuidaGene and Xinhua Hospital ophthalmology cooperation HG00401 clinical research project successfully completed the first subject administration and observed the initial efficacy, will bring more hope of cure for patients with RPE65 gene mutation-related retinopathy, but also a major progress in the exploration of the treatment of this disease in China!
HG00401 "An Exploratory Clinical Study to Evaluate the Safety and Tolerability of Gene Therapy for RPE65 Mutation in Leber's Congenital Amaurosis Type 2 (LCA2)" is an AAV-mediated gene therapy clinical study to evaluate the safety, tolerability, and efficacy of HG004. and efficacy of HG004. Currently, the clinical trial is recruiting patients nationwide, and pediatric and adult patients and their families are welcome to visit Xinhua Hospital Ophthalmology Department for consultation at 13564134543.
recommendations
-
Sep 06,2023
HuidaGene Therapeutics Announces First Patient Dosed Of The World's First Novel CRISPR/Cas13 RNA-Editing Therapy HG202 For Neovascular Age-related Macular Degeneration
-
Nov 04,2024
HuidaGene Therapeutics Receives the First-Ever FDA Clearance of CRISPR/Cas13 RNA-Editing HG202 for Macular Degeneration
-
Dec 19,2023
HuidaGene Announces Rare Pediatric Drug Designation Granted to HG302, A Novel CRISPR DNA-editing Therapy, for the Treatment of Duchenne Muscular Dystrophy
-
Aug 04,2023
HuidaGene and Kactus Announce Strategic Collaboration and License Agreement to Promote Commercialization of Next-Generation Gene Editing Enzyme, hfCas12Max®

